Semaglutide Science and Research
Let’s survey the New England Journal of Medicine study that earned Semaglutide its full FDA approval for weight loss and weight management.
Feel free to peruse the whole research report yourself (using the link above), but here are the highlights of all available semaglutide science and research:
- The more than 1900 trial participants all had a body-mass index of 30 or greater, meaning they met the clinical definition of obesity (you can calculate your own BMI using this free online tool). None of them had diabetes.
- Each participant received either a once-weekly Semaglutide dose of 2.4 mg or a placebo (a fake treatment)
- The trial lasted 68 weeks (about 1 and 1/3 years)
- At the end of the trial, the participants who received weekly Semaglutide treatments achieved an average of a 14.9% reduction in body weight; the participants who received a placebo only lost 2.4% of their body weight.
- Some participants reported nausea and diarrhea as the most common side effects, but those were generally mild and easily managed.
The graphs and charts below illustrate the differences between the Semaglutide group and the placebo (the “control”) group:
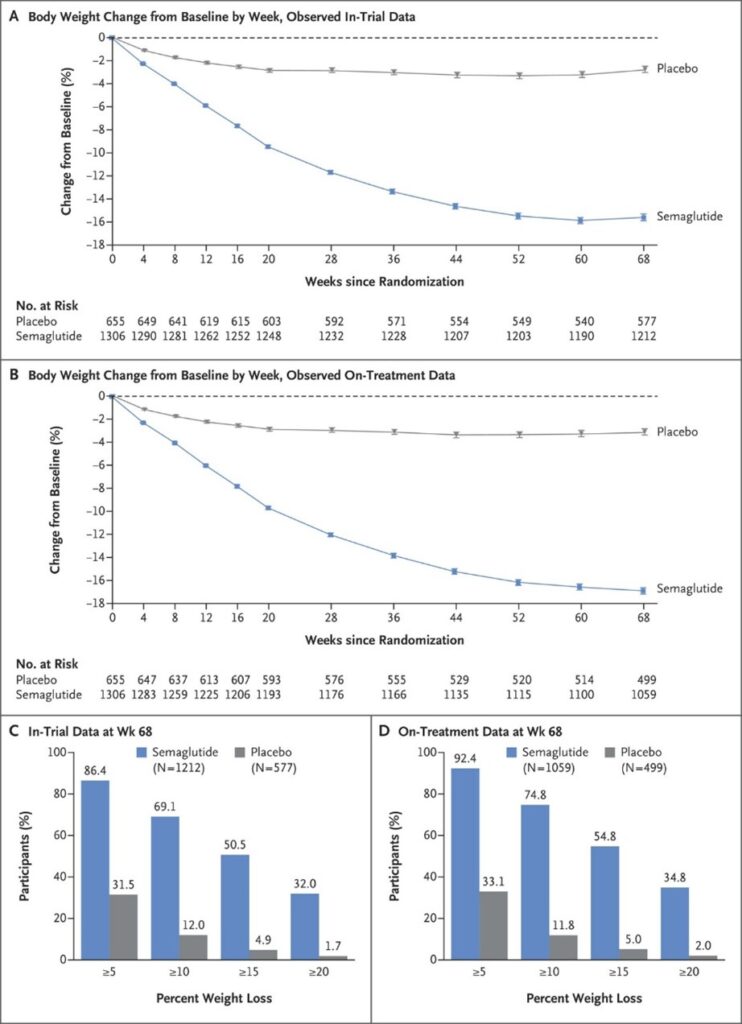
Source: New England Journal of Medicine
Another study on Semaglutide for weight loss found that, in addition to resulting in significant weight reductions, it also “increased quality of life for 40% to 50% of participants.”
Semaglutide stimulates healthy weight loss – and keeps it off long-term – through two key mechanisms:
- #1: Semaglutide triggers enhanced insulin release. The clinical research demonstrates that Semaglutide, which mimics the in vivo biological activity of the gut hormone GLP-1, “plays an important role in glucose homeostasis and augments glucose-induced insulin secretion and inhibits glucagon secretion” following food intake.
Insulin lowers blood sugar by shuttling glucose into the cells for use and storage. Stabilizing blood sugar, in turn, is a key part of controlling weight.
- #2: Semaglutide promotes satiety (post-meal satisfaction) and reduces appetite. The clinical research demonstrates that Semaglutide, which mimics the in vivo biological activity of the gut hormone GLP-1, “act[s] as a satiety factor… peripheral administration of GLP-1 inhibits food intake.”
The causal mechanism responsible for GLP-1’s satiating effects is its signaling activity on the web of hormones responsible for the feeding impulse: “In addition, interventricular injections of GLP-1 inhibit food intake, independent of the presence of food in the stomach or gastric emptying… the synergistic actions of GLP-1 in the gut and brain, acting on both central and peripheral receptors that [are] responsible for the effects of the hormone on satiety.”
Academic References
- “Semaglutide for weight loss.” Accessed via https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8589135/
- “Effects of GLP-1 on appetite and weight.” Accessed via https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4119845/
- “Appetite Regulation, Neuronal Control.” Accessed via https://www.frontiersin.org/articles/10.3389/fendo.2021.694284/full
- “Once-weekly Ozempic® mechanism of action.” Accessed via https://www.novomedlink.com/diabetes/products/treatments/ozempic/about/mechanism-of-action.html
- “Once-Weekly Semaglutide in Adults with Overweight or Obesity.” Accessed via https://www.nejm.org/doi/10.1056/NEJMoa2032183
Contraindications
Per pharmaceutical guidelines, individuals with certain health conditions should avoid Semaglutide. The contraindications for Semaglutide include:
- diabetic retinopathy (eye damage resulting from diabetes)
- impaired kidney function
- thyroid cancer
- family history of medullary thyroid carcinoma
- kidney disease (/w likely reduction in kidney function)
- pancreatitis (inflammation of the pancreas)
- low blood sugar
- multiple endocrine neoplasia (type 2)
